Background: Marginal zone lymphoma (MZL) collectively is the third most common type of B-cell non-Hodgkin's lymphoma (NHL) worldwide, with an increased trend in incidence during the last two decades (Rossi D E et al., N Engl J Med. 2022). The lack of predominant first-line systemic therapy and toxicities associated with current rituximab-based therapies have inspired the exploration of more optimal treatment strategies (Becnel MR et al., Br J Haematol, 2019). Given the crucial role of bruton tyrosine kinase (BTK) in the B-cell receptor signaling pathway, BTK inhibitor has been proven to yield robust outcomes with improved tolerability in the development of NHL (Dhillon S et al., Drugs, 2021; Xu W et al., Blood, 2019; Song Y et al., Blood, 2019). Orelabrutinib is a novel BTK inhibitor with high selectivity and has been approved in China for relapsed/refractory MZL recently (Dhillon S et al., Drugs, 2021). Meanwhile, several clinical studies reported that orelabrutinib in combination with rituximab in NHL could preserve natural killer cell-mediated antibody-dependent cellular cytotoxicity induced by rituximab and enhance the clinical efficacy (Yu H et al., Mol Ther Oncolytics, 2021; Qu C et al., Blood, 2023). However, orelabrutinib plus rituximab as the first-line treatment for MZL remains largely understudied, thus, we conducted a retrospective study to evaluate its effectiveness and safety.
Methods: Between July 27, 2021 and March 18, 2023, 10 patients with MZL who received orelabrutinib (150 mg, qd, po) with rituximab (375 mg/m 2, iv, day 1 of each 28-day cycle) as first-line therapy were included in this retrospective study. After 6 cycles of treatment, patients underwent orelabrutinib monotherapy (150 mg, qd, po) as maintenance for a year. Dynamic dose adjustment was performed according to the patient's clinical status. Effectiveness was assessed by positron emission tomography/computed tomography-based 2014 Lugano criteria. The primary endpoint was the overall response rate (ORR); secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety.
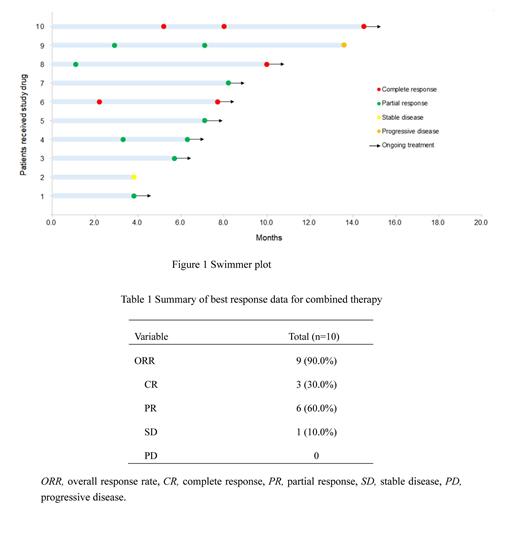
Results: Ten patients (6 males; median age 63.5 years, range 45-76) were included in this study, 4 with extranodal MZL of mucosa-associated lymphoid tissue (40.0%), 2 with nodal MZL (20.0%), 1 with splenic MZL (10.0%), and 3 with unknown subtype (30%). Among a total of 10 patients, 3 (30%) achieved complete response (CR) and 6 (60%) attained partial response (PR) as their best response for an ORR of 90% ( Figure 1). After a median follow-up of 13.0 months (range 7.8-24.7), the median PFS was not reached and a 6-month PFS rate was 100% ( Table 1). OS could not be assessed due to there were no deaths occurred. As of the cut-off date (May 6, 2023), 8 patients received orelabrutinib maintenance treatment and the median duration of maintenance treatment was 9.6 months (range 3.0-17.8). The ORR was 75% (6/8) during maintenance treatment, while 1 patient had stable disease (SD) and 1 developed progressive disease (PD). In addition, abnormal hematology due to disease gradually recovered with the therapy within 4 months. No serious adverse events were observed. The off-target related AEs such as atrial fibrillation, diarrhea, and major hemorrhage were not reported.
Conclusion: This retrospective data suggests that orelabrutinib with rituximab has an encouraging anti-tumor activity in MZL, with a favorable safety profile. These results provide a potential first-line treatment strategy in MZL. Further verification in prospective clinical trials of clinical outcomes of orelabrutinib in first-line MZL is warranted in the future.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
The indications for Orelabrutinib are as follows: relapsed/refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), relapsed/refractory mantle cell lymphoma (MCL), and relapsed/refractory marginal zone lymphoma (MZL). The purpose of this abstract is to evaluate the efficacy of first-line treatment of MZL with orellibrutinib.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal